What is immunity? How do we develop it? – Part 4 – A viral story
February 5th, 2021 | by Saren Tasciyan | posted in Biology, Immunology
If you didn’t read it yet, here is the part 1.
In the previous parts, I wrote about the different parts of the immune system. But what is immune system for, if there aren’t pathogens. Well it turns out to be there are functions of the immune system besides the fighting off an infection. But we will focus on the infection part. And in 2021 (as of the writing of this article) what other good example for an infection exists other than viruses? This will be a superficial story of a viral infection of a hypothetical virus infecting the respiratory system. Why hypothetical? Each pathogen can have unique mechanisms of infection. I don’t want to be too specific. Why respiratory system? Most common infections are respiratory system infections. More on that later in a separate article.
Imagine now a host (like yourself). Unsuspecting, doing daily biological processes. Eating, inhaling, touching. Smearing microbes from one part of the body onto another. Most of the surfaces on the body are covered with skin. This is an effective barrier between outside and inside. This physical barrier is covered with dead or dying skin cells. Not very useful for a virus, as it is an intracellular (“lives” inside a cell) pathogen. Viruses need cellular machinery. A dead cell is like a destroyed factory for a virus. Useless. Skin infecting viruses often somehow penetrate deeper to reach more alive and full of potential cells. That is now out of the scope. So skin is no good target. From outside it looks small but the surfaces “inside” of the body are quite large. Intestines are long tubular structures with lots of sub structures to increase the surface area. Also full of alive cells! Then there is stomach, mouth etc. Lungs contain many alveoli (small sacks of air, covered with cells). These also provide huge area with many living cells. And inside of your gut, stomach and lungs are considered to be outside, as there are no physical barriers to reach there. Host meets a friend. They talk 30 minutes around a table. The other host spreads tiny aerosols with each syllable into the air. These are droplets of liquid from the mouth and the throat. Some of these liquids happened to contain small particles: viruses. The other host doesn’t realize yet but some of his cells turned into factories of viruses. The immune system knows something is wrong and starting to wake up.

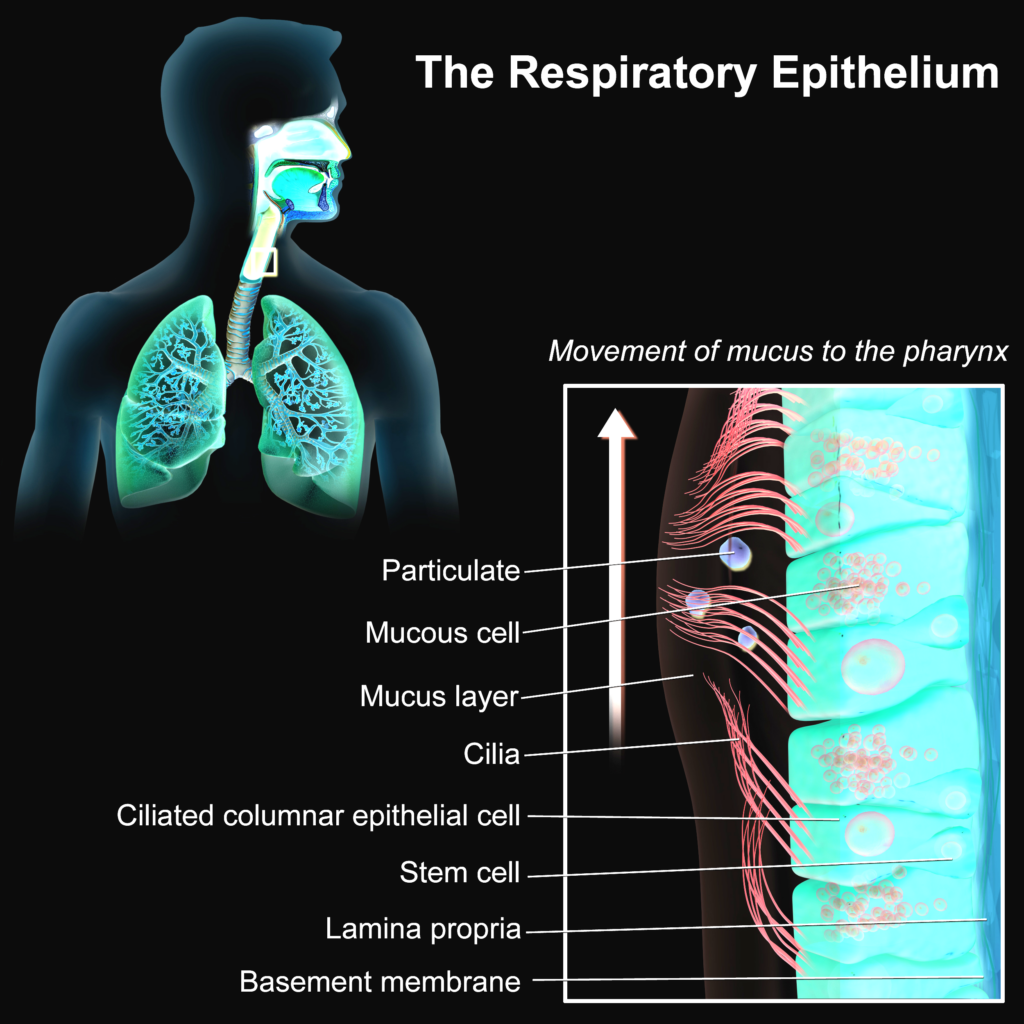
However, host’s brain isn’t aware yet. It is busy to socialize and tell a story about a joke at his workplace. These droplets spread into air and float around. Some of which, are by chance sucked into the lungs of our host. In and out, constantly aerosols move in and out. Some of these droplets manage to fuse with the wet linings of the respiratory tract. A place, where mucus slowly flows from the lungs to the throat. Not a friendly place for germs. Enzymes, peptides (very short proteins) and antibodies neutralize many foreign objects. Many virus comrades are already lost in this process. They didn’t manage. Some pioneers though, managed to randomly bump onto some host cells. It is not easy to move through the mucus towards the cells. There is a distance and mucus is viscous. And viruses don’t have any mechanism to actively move. They will bump into some cells. And that starts to happen… On different locations and slightly different times.
A few lucky viruses will actually by chance find a host cell, which they can attach to. Usually, host cells produce many proteins. Some of these proteins need to be on the cell surface. They have their functions to do certain tasks. Viruses evolve to use those proteins to get into the cell. Viral proteins have affinity to bind to specific protein(s) on the the host cell. Upon binding, viral protein changes shape. This is more of a mechanical process. For the sake of simplicity, this virus will bind to protein H (made up name) on the host cell and this will bend the viral protein to enter host cell membrane. This insertion increases the chance of membrane fusion between host and the virus. Below is an example of HIV entry into the host.

Upon membrane fusion, like 2 soap bubbles fuse, quickly the viral membrane becomes part of the host cell membrane. The viral content enters the cytoplasm.
Coming back to our story. Not all viruses, which bump into the cells are actually viable. This means that some of them are broken or incapable of entry. This can be due to mutations or protein misfolding (long chain of aminoacids make up a protein, these chains need to fold properly give a functional 3D structure). In a few locations, viable viruses managed to enter the cells… The instructions they bring are about to cause havoc.
In this one particular host cell, viral RNA is recognized by the host ribosomes (ribosomes are the machines, which make proteins from mRNA/messenger RNA). Ribosomes do what they usually do. Make proteins. They don’t know that this mRNA is not from the host itself. They blindly make the proteins. Virus tricks the host cell.
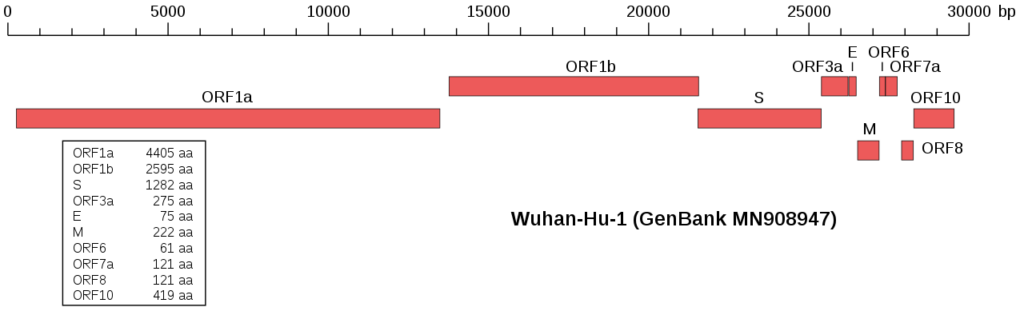
Viruses have a space issue. Their limited space doesn’t allow much of genomic content. Therefore, they are evolved to use that space very efficiently. To do that they pack all the genes they carry very tightly on their genome. Here is an example from the sars-cov-2 virus. Each red box is a different virus protein coding gene.
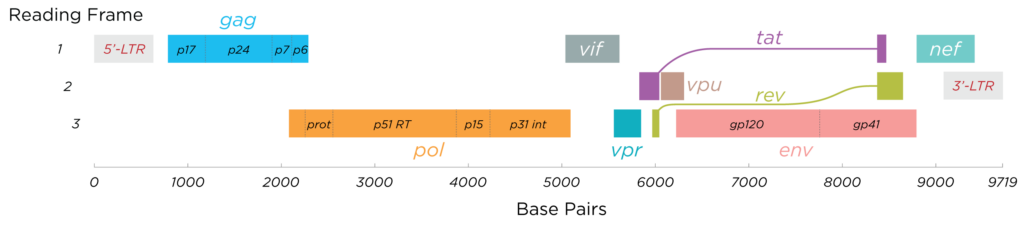
Some viruses need to have overlapping genomes. Genomes can be also read in reverse direction on the other strand. This allows some parts of the genome to have different meanings in different directions or at different start positions. Here is HIV genome as an example. For me it is amazing how evolutionary pressure can create such efficient “coding”. Programmers know this from file compression.
Meanwhile, some proteins of the host bind to the virus RNA. There are still some molecular differences. These proteins’ job is to find foreign genetic materials. This virus has just brought that. The cell is now making thousands of virus proteins. Some of these proteins are there to make the outer shell of the virus. Some are there to replicate the genome. That RNA this virus brought in need to be copied, as each viral particle needs a copy of the full viral genome. In this case similar to sars-cov-2, some of the expressed proteins are RNA polymerases. They copy the genome with the building blocks provided by the cell. With the increased number of “weird” RNAs in the host cell, some special proteins of the host bind to these somewhat foreign RNAs. They are the first mechanism to detect the infection. Proteins like RIG-I and MDA-5 do this. They detect foreign genetic material and activate the famous interferon system!
Interferon name comes from “interfering with viral replication”. Long time ago scientists realized that interferon cytokines (see part 2), interfere with the viral replication in their experiments. This is how cells
- tell other cells or even themselves that they are infected
- and tell the immune system that they are infected.
Well who said talking to yourself is weird? By the way, to this date 3 interferon types are known, based on their receptors. Type I interferons (we will speak mainly about), type II interferons and type III interferons.
| Type I | Type II | Type III | |
| Members | IFN-α, IFN-β (mainly) | IFN-γ | IFN-λ |
| Receptors | IFNAR1 and IFNAR2 | IFNGR1 and IFNGR2 | IL10R2 and IFNLR1 |
| Functions | Mainly antiviral immune response | Type 1 T cell immunity | Certain fungal and viral infections |
First infected cells produce IFN-β and this gets secreted. Our virus has a gene, which interferes with interferon signaling. An interferinterferon! Ok I made this name up but such genes exist to interfere with host detection mechanisms, such as the interferons. Our virus in this case has a NS1 (nonstructural, inspired by true events, see below 🙂 ) protein, which binds to viral RNA to protect it from RIG-I. NS1 also prevents host cell mRNA functions, which is required to produce interferon response. It doesn’t end here, it also interferes with RIG-I signaling pathway. One protein with so many functions… A real life example is the NS1 protein of an actual influenza virus (Li et al. 2020).
Now, our host launches a defense system, which is reduced by the virus. Meanwhile, this host cell became a virus factory. Thousands of viruses are released and they have a good advantage of a head start in a new race. Released viral particles aren’t infectious immediately. Once outside, they maturate. These are changes on their structure. They kind of get ready. This is to prevent immediate binding to the host cell they have just exited. Host cell meanwhile is sick and there are immune cells, which recognize that. This triggers another set of immune responses. Some sick cells undergo apoptosis (cell suicide). This leaves clues for the immune cells. They pick up the pieces and eat them (phagocytosis).

Then they process these units into small chunks and move to lymph nodes to display it (on MHC-II) to T and B lymphocytes to find a random match. At the same time, sick cells do the same thing internally and present their content on their surface (MHC-I). These processes are called antigen presentation. DCs may also take up antigen, process it but display on MHC-I like other non-immune cells to present the antigen to the special type of T cells, so called cytotoxic T cells. This special type of T cells are there to kill specifically infected cells to reduce viral load thanks to their specific TCR and enzymes that destroy cells.
Coming back to our story (again). At this point virus couldn’t escape the host immune activation completely. The mechanisms it relies on spreading also exposed it to the immune system (as usual). Now, the immune system tries to buy time with innate immune defense actions to develop a more specific and effective immune response against the invading pathogen. Most importantly, the interferon system already warned the other cells of the infection. Healthy cells change their gene expression to be more resistant to a viral infection. Some DCs already picked up dead cells and they are on their way to lymph nodes. They also picked up the danger signals due to the tissue destruction, which they will present to naive T cells and naive B cells. Naive T/B cells are cells, which survived the initial selections (they are functional and wouldn’t attack the host itself) but they don’t know yet if they were lucky enough to recognize any pathogen. DCs circle around millions of T/B cells in the lymph nodes hoping to find a T and B cell, which can recognize the pathogen. Meanwhile, fluid also runs from the tissue through lymph vessels into lymph nodes, where it gets filtered. Smaller proteins manage to enter the lymph node. If lymphocytes recognize them, they also get activated. Due to the activated immune functions the host feels fatigue, headache and pain. The lymph nodes are swollen to increase the chances of finding the right lymphocytes.

Virus keeps expanding. Innate host defenses are unmatched with the speed of viral spread. Millions of cells are dying or producing more viruses. Some of these viruses are secreted with each talk/cough to find new hosts. Many will fail but all it takes to find at least one more host to keep going. With increased tissue damage more and more destructive cells come to the site of infection. Most badass type of cells are neutrophils, which may not be the most suitable cell type for a viral infection. They cause even more damage at a desperate attempt to stop the infection. But also macrophages arrive, which clean up the mess by eating almost anything damaged. Hosts’ fever goes up. It is not clear why fever even exists. Treating it doesn’t worsen the outcome but it helps the immune system in various ways. Immune cell migration and T cell proliferation increase with fever.
In the lymph node, while a mature DC (they maturate after taking up antigens) was moving between T cells, it begins to interact with one of the T cells longer than usual. Normally, these interactions are short-lived but this T cell doesn’t let the DC go. Multiple proteins on the surface from both cells adhere to each other to serve as a probing platform to the antigen. Each protein-protein interaction has their own functions but the most important one is the TCR – MHC-II interaction. Each T cell has a slightly different TCR. As this T cell has now started several signaling cascades, it transforms into an activated T helper cell. The danger signals DC provides prevent it to be a regulatory T helper cell (the kind that suppresses immune response). Instead, it is about to coordinate whole sorts of immune responses. First, it needs to divide (clonal expansion). The ones with highest affinity has also strongest signal to proliferate and survive. This way the immune system favors better receptors against the antigens. A similar but yet different development occurs at the B cell side.

Innate immune system was losing the battle. It causes lots of collateral damage into tissues. Slowing down the virus simply isn’t enough as viruses are usually much faster. But the site of infection calls many cells with the help of the cytokines and chemokines. Some of the generated T and B cells leave the lymph node and circulate in the blood. They once in a while bump into the blood vessel but that doesn’t stop them. Except for certain blood vessels. In this case these are the blood vessels of the site of infection. These are particularly sticky for these cells. First, a weak adhesion to selectins causes the cell to roll on the blood vessel walls. Then a stronger adhesion is formed and the T and B cells can stop and squeeze themselves through the cell-cell junctions of the epithelial cells of the blood vessel. This process is called extravasation. Further chemotactic cues within the tissue guide the lymphocytes to the site of infection. Some of these cells are the cytotoxic T cells. They recognize infected cells due to MHC-I-antigen complexes. These cells report themselves to have the virus antigen. Cytotoxic T cells destroy them specifically, as their TCR binds strongly to the MHC-I+antigen. Some viruses try to prevent this by disabling MHC-I. Then natural killer cells kills those cells for not having any MHC-I. This is like being arrested for not carrying any ID. Most importantly, things are about to change as T cells joined the fight. There is not much escape from the immune system.
B cells can be activated either without the help of T cells (T cell independent antigens, hereafter TI) or with the help of T cells (T cell dependent antigens, TD). TIs are mostly molecules other than proteins , whereas TDs are mostly proteins (for simplicity of this article). Naive (doesn’t know if it will recognize any antigen yet) B cells in the lymph nodes can take up proteins if their BCR binds to an antigen and process them similar to DCs. Likewise, they also present it on their MHC-II. A T cell, which is already activated can find this B cell and activate it, because this T cell have also the ability to bind the same peptide/antigen. This interaction contains other information. T cell presents ligands (proteins that are signals) on their surface and secretes cytokines. B cell catches these signals and from now on this is an active B cell. An active B cell’s strongest weapon/function is to produce those famous antibodies. First class of antibodies produces are IgMs. These are group of 5 antibody units in one molecule, as the first antibodies may not be that strong. B cells meanwhile, divide to produce more cells, in which they randomly slightly change the recipe for the antibodies they produce. Some recipes decrease the quality of the antibody but some increase it. The ones that have a better quality (stronger binding to the pathogen) also receive higher chance of survival. Also with better antibodies they switch from IgM (5x) to IgGs (1x). B cells turn into antibody factories. Now body has finally gained the upper hand in this battle. This response is faster than the viral replication.

Any released virus is almost immediately covered with antibodies, as they bind like magnets. Viral particles are unable to reenter any cell and also they are eaten up by immune cells. Complement system helps with the destruction of each viral particles. Some antibodies bind to infected cells to call the immune system for their destruction. Remnants of dead cells are cleared up by macrophages and injured tissues start to repair. With the decreased of damage and viral particles, immune systems starts to decrease inflammation. Many lymphocytes start to die as there is no need for them anymore. Only few B and T cells are lucky to survive and become memory cells. They will wait for a long time until this virus is back. This whole mechanism is so amazing and efficient that enables larger animals like us to survive viral infections. Without it even a common cold can become a deadly disease. It even simulates an evolutionary model within a single individual. B lymphocytes mutate to make better receptors and antibodies. Best ones proliferate (divide) more than the others. But it also comes with a price. I will discuss this in autoimmunity part.



