What is immunity? How do we develop it? – Part 1 – Cells
January 10th, 2021 | by Saren Tasciyan | posted in Biology, Immunology, Science
Let’s start with a cliche sentence: 2020 has been a challenging year for many of us. COVID-19 is continuing to spread all over the world. On countries level, we had cycles of ups and downs of epidemic curve. At the end of 2020, there was a light of hope to get out of this cycle of waves. 2 vaccine candidates were approved. While many debate the vaccines on the social media, understanding of the biology behind it and how the immune system works is mostly absent. This is normal. Immunology is a complicated topic (you will see why) and if you aren’t dealing with it on a daily basis, you won’t be exposed much to the information. The goal of this article is to give a superficial idea of the immune system and its mechanisms. Spoiler alert: it will start to get very confusing with lots of terms and it will hopefully get clearer towards the end. So please bear with me. For the experts: this article is a trade of between factual correctness and conciseness. If you think, you can correct something about the article, please don’t hesitate to contact me. Please bear with the content. I promise a lot of things you read will make more sense at the end with the given information. This is part 1 of a series of blog posts.
I find immunology extremely fascinating and that is why I studied it. A system that is so smart yet without a brain is amazing. Even more interesting is the evolutionary mechanisms, which led to this.
Immune system is very old (evolutionary) and I will focus on the human (although it is very similar with close relatives such as apes, rats, etc.). The whole idea of it to clear pathogens (harmful viruses, bacteria, parasites, fungi) and develop defenses against them, so that a next exposure isn’t as harmful as the first one. In terms of evolution it has been a constant battle between pathogens (harmful germs) and us (hosts). Believe it or not, we are winning.
It is VERY important to remember that the immune system is sort of divided into 2 parts: innate and adaptive. Innate immune system doesn’t really learn much from diseases. It is more like an automatic system, which clears the pathogens. There isn’t a drastic improvement at the second exposure. Adaptive immune system actually “learns” from previous exposures. This was an enormous evolutionary advantage. This learning is accomplished by recognizing the surfaces of pathogens and it doesn’t involve your brain. It isn’t like learning in traditional sense. This enables two things:
- You have some defenses instantly ready to fight
- You have defenses ready to be deployed more rapidly (there is a slight delay)
Probably, most important category of actors of the immune system are cells. We have many cell types. I will mention only most relevant ones.
Macrophages are large cells, which like to eat a lot. They are rather part of the innate immune system. When they see a bacteria wondering around, or a virus covered with antibody, they attach them and engulf them. After this the pathogen is kept in a vesicle (like a cell membrane but inside of a cell). This vesicle is transported and fused with other vesicles containing enzymes, which degrades the contents. Also pH is lowered to dissolve better. Literally, they eat pathogens. Actually it is in the name: macro (big) phage (eat). They also clear up mess such as dead cells.
Dendritic cells are part of the innate immune system but they are also a bridge to adaptive immune system. They collect materials from the environment (self means materials from the host itself, foreign means materials not coming from the host). They then enter the lymphatics to travel to lymph nodes, where they present the processed stuff to adaptive immune system. They do this by providing bunch of additional signals. This is important for the immune system to know if the presented material is associated with danger or safety. The way they do this to collect information from the environment of the sample collection. Are there signals, which are associated with danger? (DAMPs: danger associated molecular patterns or PAMPs: pathogen associated molecular patterns) Then these cells process this information by chopping the collected proteins and present them on MHC (major histocompatibility complex).
Neutrophils are the badasses of the innate immune system. They are extremely destructive. They are the first line of defense after a breach. They move rapidly to the site of infection or injury. Once they see things like bacteria or fungi, they release bunch of stuff. Some of which are lysing (molecular digestion) the pathogen. They also cause collateral damage into surrounding tissues. If they are REALLY triggered, they undergo a suicide missions called NETosis. This is a particularly interesting process, where the cell dies intentionally to release all of its DNA to catch and immobilize pathogens. This also signals to the immune system that something really nasty going on at the site of infection. They sort of suicide bomb the site. They are also very well migrators (cell migration is the process of cells moving in an environment actively). Their nucleus (stiff globular compartment, which holds the DNA) is fragmented, which is thought to let them migrate through narrow constrictions easily. They are so destructive that they are really short lived. They live roughly a few days and then get eaten up and removed. But they also are the most abundant immune cells in the blood. For this, our bone marrow produces a lot of them constantly. When we get old, bone marrow gets “tired” (cellular aging) and can’t produce as much. If you get chemotherapy, dividing cells (bone marrow) gets killed and can’t produce as much. These results in susceptibility to infections. Here is famous old video of a neutrophil chasing a bacterium.
T cells/T lymphocyte are like commanders of the adaptive immunity. There are different types but in general they are amazing. They start their life as a progenitor cell (they are like new soldier without any assigned function) and their most important weapon is their TCR (T cell receptor). TCR is supposed to recognize/bind any new potential pathogen surface. But how could it know that? How can you make a molecule that binds to something, which isn’t there yet?! Pathogens evolve. They change their surface to evade host detection. We are worried that coronavirus (sars-ncov-2) mutates and evades our immunity causing a second pandemic. How can the adaptive immune system be ready in advance? Well, they kind of evolve too. Here is the idea. T cells during development randomly change their DNA in several ways. They combine different parts, they mutate (change) the generic code to diversify TCRs in each cell. But each cell need to have one type of TCR. The idea is to have many cells with different TCRs. Potentially, one will recognize sars-ncov-2 or any other pathogen. The problem with this approach is to not accidentally recognize something of our own (host). When that happens the immune system attacks the host itself. To avoid this, T cells after “birth” (technical term: differentiation) in the bone marrow travel to thymus via blood vessels. They then present there if their TCR is functional at all. All those changes may break the TCR completely. Like a quality control in a factory. Or more like an initial selection for navy seals. If TCR can bind to a mock MHC (aiming target in a firing range), that T cell gets to live. Otherwise, they need to die. This is called positive selection. Then the next test they need to succeed is not to recognize self. They are presented with a variety of host’s own proteins. If they accidentally recognize one of these and get activated, they get killed too. This is called negative selection. This is like those military trainings, where targets pop up in the area, some of which are terrorists and others are civilians. You don’t (hopefully) get to be a soldier if you shoot civilians. This is exactly the idea.
After this T cells are considered to be finished with their “training”. Failed cells die. Successful ones wanders around the body from one lymph node to another waiting to recognize something. When they do, they can either organize immune response as T helper cells or become cytotoxic T cells, which go and kill infected host cells. Ah also there are memory T cells. Which live REALLY long. Like years long! They remember their target and wait until they show up again.
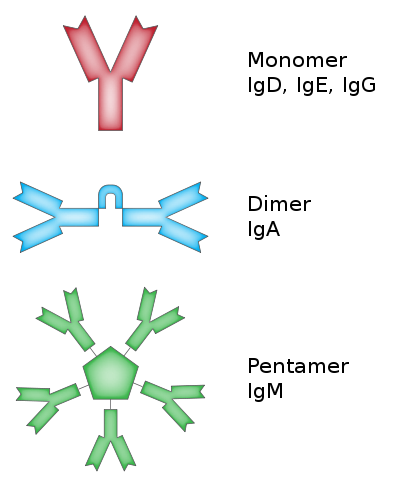
B cells/B lymphocytes are similar to T cells. However, instead of TCR they have BCR (B cell receptor – not so creative). They similarly increase variation of BCRs in each B cell and undergo positive and negative selection. All of this effort is to avoid autoimmunity. A disease, where host immune system attacks the host itself. B cell response is a bit more different than T cell response. B cells are particularly known to produce antibodies. These are proteins, which bind to their target (such as a virus protein) and neutralize. More of which we will talk about later. See, BCR is on the B cell surface and it is fixed there. But antibodies we know are soluble in bodily fluids freely diffusing around. To do that an activated B cell becomes a plasma B cell. These cells are antibody factories. They even produce different kinds of antibodies for different purposes and regions. First response is IgM (5 antibodies in a chain). During this B cell continues to mutate BCR to try to make better antibodies (stronger binding). Those by chance with better antibodies survive and live longer. Then they start to produce IgG. There is much more about this topic but this part is relevant for brevity. Like memory T cells, there are also memory B cells. These cells also live very long and wait until their target shows up.

T and B cells are the main cells of the adaptive immune system. They create diversity to be ready for all the unknown mysterious germs of the world. The problem is the first exposure. It takes time to get the right cells activated. This time can mean life or death for the host. Once the cells are activated and memory is formed, they are ready for the next deployment. This can cut down days, in which the germs are spreading from one cell to many others. That’s why having immunity to a pathogen makes a big difference. Additionally, circulating antibodies can immediately neutralize incoming antigen (antigens are molecules which are targets or potential targets of the immune system, self antigen is a molecule of the host). But you will have to have the immunity to that antigen first. Adaptive immunity isn’t perfect though and it comes with a cost. We will discuss this later.
Now that you know the cells of adaptive and innate immunity, it is time to talk about how they talk to each other. Dendritic cells (DCs) take up the antigens and process these. A chunk of the processed antigen is presented on the MHC. DCs after taking up antigens enter lymphatic vessels (another vessel system separate from blood vessels but these collect liquid from tissues and directs this liquid towards the lymph nodes. Lymph nodes are organs full of immune cells). DCs travel to lymph nodes via lymph vessels. There they present these signals on MHC to T cells. MHC and TCR bind each other. In parallel DCs present signals to indicate danger or safety. Based on these signals and the binding affinity T cell decides to:
- ignore OR
- become activated to organize an immune response OR
- become activated to tolerate the antigen.
This connection between DC and T cell is called immunological synapse.

Well that’s enough for one article. In the next article I will describe, how do these cells communicate with each other.
By the way, some of these images are from Pedro Veliça. He has an amazing nerdy science artwork collection. Don’t forget to have look at his gallery.



