These uranium atoms are “excited” for the decay of their peers
February 25th, 2024 | by Saren Tasciyan | posted in Electronics, Science, Technology, Video

About a year ago, I found myself engaged in a conversation with a seller in a quaint alley. As he presented the piece, I swiftly retrieved a device from my backpack, commencing with measurements. To my delight, the device responded with a series of affirming ticks. Seeking further confirmation, I produced a specialized lamp, and sure enough, the results were conclusive.
With satisfaction, I extracted the necessary funds and carefully stowed the piece in my backpack, crossing my fingers that it would withstand the journey without turning into fine dust. The seller then inquired if I had an interest in another piece, sparking excitement within me. After a brief absence, he returned with the second piece. I eagerly pressed my device against the metal, and the familiar tick tick tick echoed.
The uniformity of the ticks made it impossible to distinguish individual beats. Regrettably, this particular piece was not available for purchase, yet the experience of witnessing it in action was undeniably enjoyable.
This seemingly shady narrative is, in fact, entirely legal. The intriguing pieces involved in this tale are a fragment of green-looking glass and a watch. The green hue is a deliberate attempt to challenge a common misconception about uranium. \

While uranium metal itself does not typically glow green, adding just a small amount (~0.1 – 2% w/w) of it to molten glass and cooling it results in a mesmerizing poison-like green color. The particular piece discovered is a perfume bottle with a makeshift lid, which itself lacks uranium content and exhibits a more yellowish hue.
Despite the low radiation levels, cautious long-term storage near oneself is not recommended. α-particles travel only a few centimeters in air, while β-particles can cover a few meters. Historically, such glass items were common, and people even used them for eating and drinking. The uranium being trapped in the glass and the low radioactivity rendered them relatively harmless. Unfortunately, the production of such beautifully crafted glass items has decreased over time, making them rarer to find today. However, caution is advised, especially when dealing with the fine dust resulting from breakage.
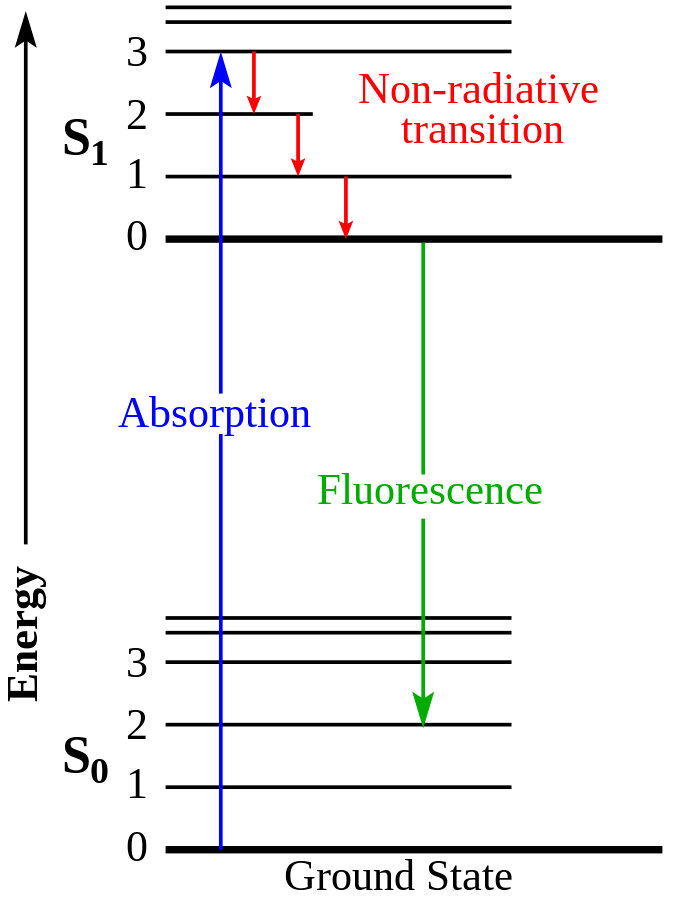
But there’s more to the story. Uranium glass exhibits fluorescence under black light or UV light, emitting a captivating green glow. This phenomenon is unrelated to radioactivity but rather stems from a physics phenomenon known as fluorescence. The absorbed light excites electrons, which, upon returning to their original state, emit light. This glow-in-the-dark effect is distinct from radioluminescence, where radioactive decay directly excites phosphor atoms, as seen in the radium/phosphor paint of the aforementioned watch.
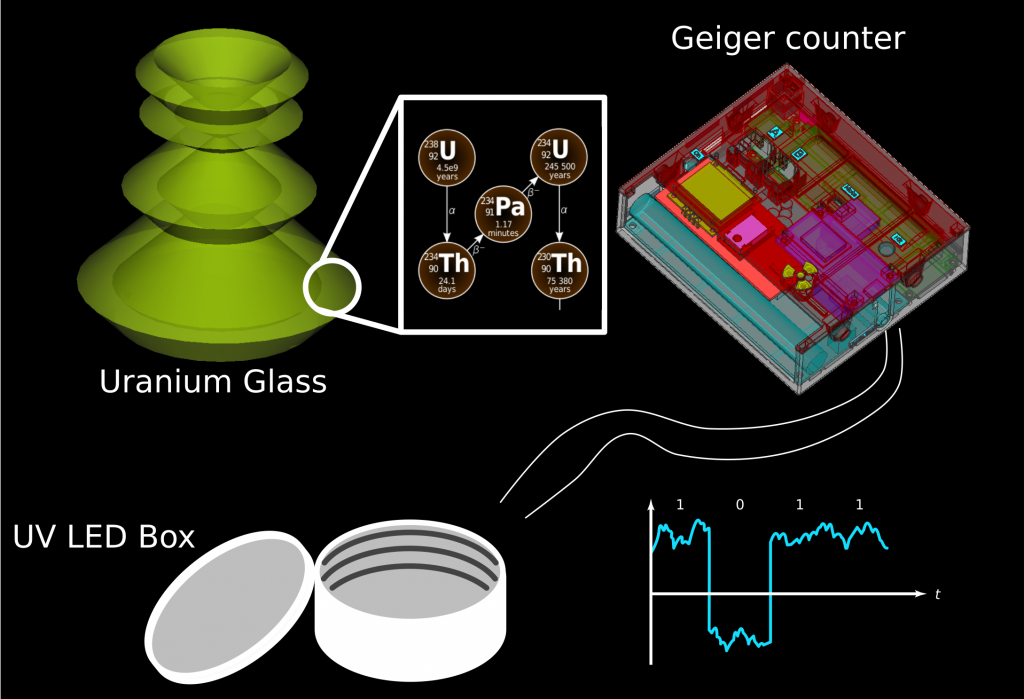
With both radioactive and fluorescent properties in mind, an idea emerged. Utilizing a self-made smart Geiger counter, based on RadiationD-v1.1 (CAJOE), equipped with a port that electronically signaled each tick, a setup was devised to control a UV light box. The UV light box, originally intended for curing 3D prints, featured a timer controller. With this I should have been able to glow the uranium glass with every particle detection.

The setup encountered a challenge related to the signal issue inherent in the Geiger counter’s design. The default state of the signal was high, meaning that it was at a constant voltage when not disturbed. In a typical Geiger counter, when a particle strikes the Geiger tube, it induces a discharge, leading to a drop in the output voltage. However, for the intended purpose of turning on the UV LEDs upon particle detection, the default high signal posed a problem.
To overcome this hurdle, I quickly put together a simple solution in the form of a straightforward voltage inverter. This inverter featured an NPN-type BJT (Bipolar Junction Transistor). The purpose of this inverter was to alter the default high signal to a low state during steady state. When a particle triggered the Geiger tube, creating a noticeable drop in the output voltage it created a high peak in the signal. The UV LEDs needed to be activated upon detection with high signal.

Here is the detailed explanation of what happens:
- Within the uranium glass, numerous uranium cores and their decay products undergo decay, releasing α and β particles in the process.
- By sheer chance, some of these particles (mostly β in the case of my cheap Geiger counter) manage to reach the Geiger tube, instigating a discharge that results in the release of roughly a 600 V charge within the tube.
- The internal electronics of the Geiger counter meticulously process this event into a digital signal. By default, the signal is high, set at 5 V, and drops to low (0 V) when the tube discharges.
- When the Geiger counter’s output voltage is high, it activates the transistor, causing the voltage at the output to be equal to ground (0 V). This occurs because the voltage drops across the R1 resistor. Conversely, when the Geiger counter’s output voltage is low, the transistor is off, and the voltage at the output matches VDD (5 V). The R2 resistor serves to limit the current over the gate of the transistor.
- Whenever the output voltage after the signal inverter is high (5 V), the MOSFET is turned on. This allows current to flow to the LEDs, generating UV light. In this setup, MOSFETs, though a type of transistor, are specifically chosen for their efficacy in controlling power applications. Given that LEDs consume a substantial amount of energy, the MOSFET plays a crucial role in directly regulating their operation.
- The emitted UV light from the LEDs serves as the excitation source for electrons within the uranium glass. Subsequently, these excited electrons return to their original state, emitting green light in the process.
- Then the cycle repeats with the next detection.
Here is the final result of a fun evening activity:
Sources for some of the illustrations:
- https://commons.wikimedia.org/wiki/File:Decay_Chain_of_Uranium-238.svg
- https://commons.wikimedia.org/wiki/File:Digital-signal-noise.svg
- https://commons.wikimedia.org/wiki/File:TO-92_Back.svg
- https://commons.wikimedia.org/wiki/File:Jablonski_Diagram_of_Fluorescence_Only-en.svg
- https://www.quora.com/Why-does-Homer-handle-a-rod-of-uranium-in-the-opening – Homer in the title sequence of “The Simpsons”, copyright 20th Century Fox.



